About Lasix ONYU
What is Lasix ONYU?
Lasix ONYU is a prescription drug-device combination that treats edema caused by fluid build-up for adult patients with chronic heart failure. It is designed for subcutaneous administration and intended as an at-home treatment option for eligible patients.
Why Lasix ONYU?
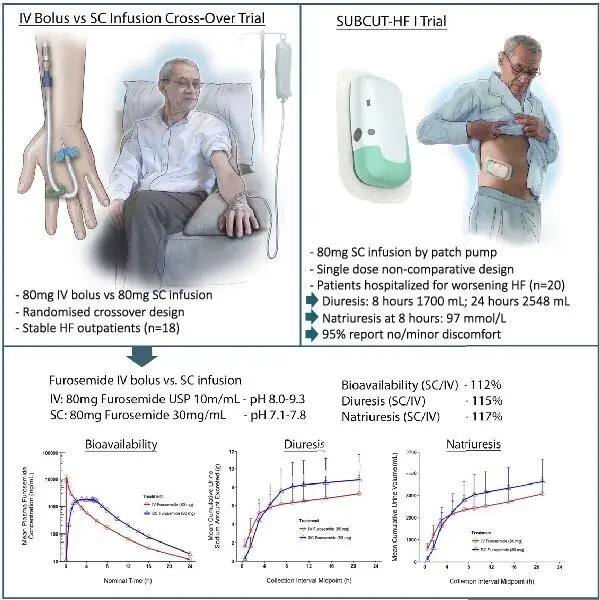
Lasix ONYU was designed to address challenges in managing edema outside of the hospital. In patients with edema, gastrointestinal edema can impair the absorption of oral diuretics, reducing bioavailability and limiting therapeutic response. Lasix ONYU delivers a high-concentration formulation of furosemide via subcutaneous infusion, achieving 112% absolute bioavailability relative to intravenous administration¹.
Subcutaneous administration enables effective outpatient diuresis without the need for IV access.
Lasix ONYU is available through emergency departments, outpatient clinics, and leading distributors, to ensure wide access. While it may be prescribed and dispensed in the ED or outpatient setting, treatment is intended to begin at home.
This delivery model supports flexible clinical workflows by enabling patients who do not require hospital admission to receive timely treatment at home. It also facilitates transitions of care and aligns with current efforts to expand outpatient management of heart failure.

¹ Osmanska J, Brooksbank K, Docherty KF, et al. A novel, small‑volume subcutaneous furosemide formulation delivered by an abdominal patch infusor device in patients with heart failure: results of two phase I studies. European Heart Journal ‑ Cardiovascular Pharmacotherapy. 2024;10(1):35‑44. doi:10.1093/ehjcvp/pvad073
The following information is for informational purposes only and is not intended to replace the Instructions for Use.
Read the full Instructions For Use and Prescribing Information prior to taking Lasix ONYU.
How Lasix ONYU Works
Drug Formulation
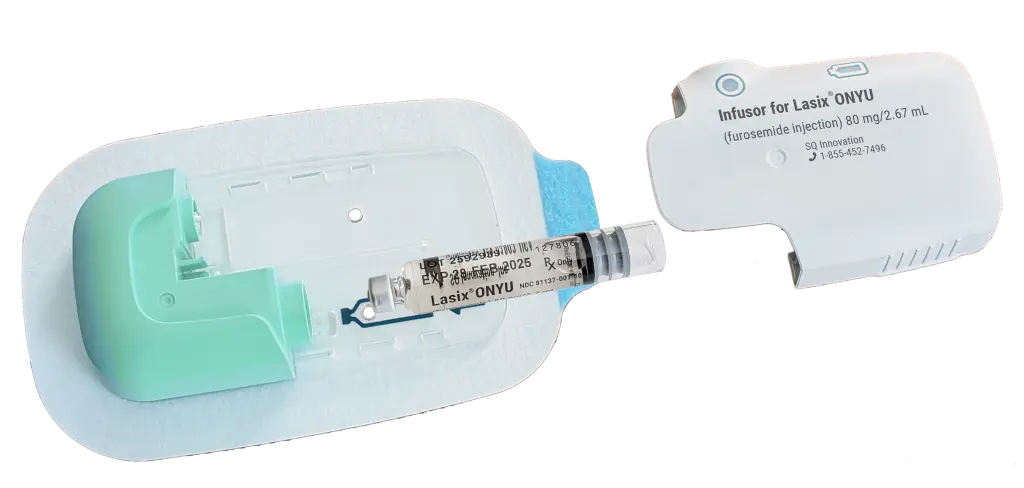
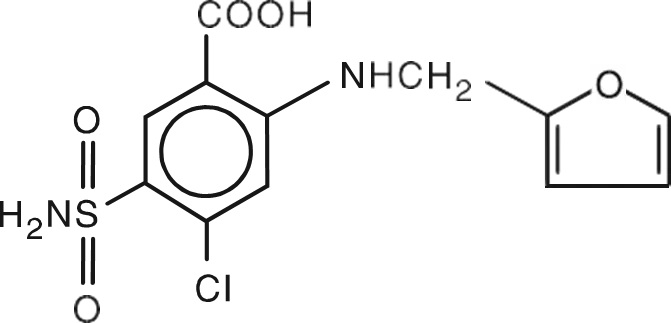
Lasix ONYU contains furosemide, a well-established loop diuretic, in a novel, pH-neutral formulation concentrated at 30 mg/mL. Each prefilled cartridge contains 80 mg of furosemide in 2.67 mL. The formulation includes betadex sulfobutyl ether sodium and other excipients to enable subcutaneous administration and is formulated to reduce the potential for discomfort at the infusion site.
The Infusor Delivery System
Lasix ONYU is administered using a wearable, on-body Infusor composed of two components:
A Disposable Unit (DU) that is sterile, single-use component that houses the drug cartridge, needle, and adhesive patch
A Reusable Unit (RU) that powers and controls the infusion process and can be used for up to 48 treatments
Once the Infusor is applied to the abdomen and activated, it delivers 80 mg of furosemide over 5 hours. The pre-programmed delivery profile—30 mg in the first hour followed by 12.5 mg/hour for the next 4 hours—is designed to provide gradual, sustained diuresis over several hours.
Treatment Profile
By enabling subcutaneous administration in an outpatient setting, Lasix ONYU offers an alternative for patients who require diuretic therapy but may not require hospital admission. The delivery profile is designed to help reduce the intensity of diuresis associated with rapid intravenous infusion, while still achieving the desired therapeutic outcome.
Lasix ONYU may help eligible patients manage fluid overload while remaining at home, under the guidance of a healthcare provider.
Clinical Data
In an open-label, randomized crossover study, Lasix ONYU produced completed bioavailability (112%) when compared to same dose of intravenous (IV) bolus furosemide.
In the same study, diuretic response was comparable between the two treatment groups. At 8 hours, mean urine output was approximately 2.80 liters with Lasix ONYU, versus 2.35 liters with intravenous (IV) bolus administration. At 24 hours, urine output was 3.65 liters with Lasix ONYU, compared to 3.09 liters with IV bolus.
Comprehensive pharmacokinetic results from this study are available in Section 12.3 of the Prescribing Information
About the Name: Lasix ONYU
Lasix® was first introduced in 1964 as the brand name for prescription products containing furosemide. It has been used in the United States and other countries over several decades. In 1982, furosemide became available as a generic medication in the U.S., and as with many medications, the generic name became more commonly used in clinical practice over time.
Despite this, the Lasix® brand name has remained recognizable in many settings. Lasix® ONYU reflects a new drug-device combination product containing furosemide for subcutaneous use. The term “ONYU” is used as a modifier to differentiate this formulation from other forms of furosemide, such as oral or intravenous. The name is intended to help healthcare professionals distinguish this product for its specific route and setting of administration.
Novel Furosemide Formulation
The trademark LASIX® is registered for Validus Pharmaceuticals L.L.C. in the United States and used by SQ Innovation under license.

